Air conditioner design
Refrigeration systems contain high pressure gas, some of which can break down into poisonous gasses when heated. The voltages used are lethal. If you are not confident, call a professional
A short description of air conditioning
An air conditioner is not a source of cooling but a pump that moves heat from inside the structure to outside where it is unobjectionable.
The theory of operation of these machines is a bit more complicated.
To massively oversimplify, I can change the boiling point of any liquid by changing the pressure on it.
By using a compressor to change pressures I can make refrigerants boil and condense at the temperatures that I want.
When the refrigerant boils at low pressures it absorbs heat from the structure.
Then the refrigerant condenses at high pressures outside the structure releasing the heat it absorbed inside the structure. Then the cycle repeats.
There are some limitations on what the layman can do involving refrigerants as they cannot be sold to unlicensed persons.
The theory of operation of these machines is a bit more complicated.
To massively oversimplify, I can change the boiling point of any liquid by changing the pressure on it.
By using a compressor to change pressures I can make refrigerants boil and condense at the temperatures that I want.
When the refrigerant boils at low pressures it absorbs heat from the structure.
Then the refrigerant condenses at high pressures outside the structure releasing the heat it absorbed inside the structure. Then the cycle repeats.
There are some limitations on what the layman can do involving refrigerants as they cannot be sold to unlicensed persons.
Below are 2 slide shows to illustrate the theory of heat
| introduction_to_heat.ppt | |
| File Size: | 2215 kb |
| File Type: | ppt |
| intro_slide_show1.ppt | |
| File Size: | 2799 kb |
| File Type: | ppt |
Latent heat and sensible heat.
Sensible heat. This type of heat is easily measured by a thermometer. An example is water from a faucet in a sink. If I turn on the cold water and place my hand in the stream, I can feel the temperature. If I then turn on the hot water and place my hand in the stream, I can feel the temperature difference. When the cold water passed through the water heater its temperature was raised using sensible heat. Water uses 1 BTU per Pound to raise it one degree fahrenheit.
Latent heat is the heat absorbed by the change of state of a substance. It cannot be detected with a thermometer.
When a pot of water is placed on a stove burner, the water will begin to boil. as the water boils, it does not change temperature. No matter how much heat is added, the temperature of the water stays the same. The water just boils faster. It absorbs heat to change the water into steam. When the water has boiled away, the pot gets very hot and may warp or melt. The reason the pot heats up after the water is gone is there is no latent heat being absorbed. (see the latent heat experiment below)
When water is in solid form, it is ice. Ice can be very cold or as warm as 32 degrees F. Once it reaches 32 degrees, it can get no warmer than 32 degrees and still be ice. You can apply as much heat as you like but the ice will stay the same temperature until it changes to water. Ice has to absorb 144 BTUs of latent heat per pound to change to water.
After the ice is water, it again absorbs sensible heat. I feels warmer as the temperature rises.
When the temperature rises to 212 degrees F it can get no warmer and still be water. It again must absorb latent heat to change state to steam. This time, the latent heat of vaporization is 970 BTUs.
After it has all changed to a gas, it again absorbs sensible heat.
Now lets reverse the process.
Steam at 230 degrees F, if exposed to lower temperatures, will reduce in temperature until it reaches 212 degrees F. At that point, the temperature will stay the same until it changes to a liquid.
Remember that 970 BTUs? When the steam changes state to water, the 970 BTUs are released. This called the latent heat of condensation.
I absorbed 970 btus of latent heat to boil my water and that heat was contained in the steam. When I cooled the steam, it condensed and released the latent heat that was absorbed when it boiled.
The same is true for the change from water to ice. The 144 BTUs must be released to change the water to ice.
This property of liquids and gasses is the basis of air conditioning and refrigeration. This is why we say we are moving heat not creating it.
This concept must be fully understood to before proceeding.
Latent heat is the heat absorbed by the change of state of a substance. It cannot be detected with a thermometer.
When a pot of water is placed on a stove burner, the water will begin to boil. as the water boils, it does not change temperature. No matter how much heat is added, the temperature of the water stays the same. The water just boils faster. It absorbs heat to change the water into steam. When the water has boiled away, the pot gets very hot and may warp or melt. The reason the pot heats up after the water is gone is there is no latent heat being absorbed. (see the latent heat experiment below)
When water is in solid form, it is ice. Ice can be very cold or as warm as 32 degrees F. Once it reaches 32 degrees, it can get no warmer than 32 degrees and still be ice. You can apply as much heat as you like but the ice will stay the same temperature until it changes to water. Ice has to absorb 144 BTUs of latent heat per pound to change to water.
After the ice is water, it again absorbs sensible heat. I feels warmer as the temperature rises.
When the temperature rises to 212 degrees F it can get no warmer and still be water. It again must absorb latent heat to change state to steam. This time, the latent heat of vaporization is 970 BTUs.
After it has all changed to a gas, it again absorbs sensible heat.
Now lets reverse the process.
Steam at 230 degrees F, if exposed to lower temperatures, will reduce in temperature until it reaches 212 degrees F. At that point, the temperature will stay the same until it changes to a liquid.
Remember that 970 BTUs? When the steam changes state to water, the 970 BTUs are released. This called the latent heat of condensation.
I absorbed 970 btus of latent heat to boil my water and that heat was contained in the steam. When I cooled the steam, it condensed and released the latent heat that was absorbed when it boiled.
The same is true for the change from water to ice. The 144 BTUs must be released to change the water to ice.
This property of liquids and gasses is the basis of air conditioning and refrigeration. This is why we say we are moving heat not creating it.
This concept must be fully understood to before proceeding.
Pressure of gasses

We have 2 ways of measuring gaseous pressure.
Absolute pressure (PSIA) assumes we are in a complete vacuum. Many things in this business use absolute because it the basis of all pressure.
Gauge pressure (PSIG) starts at the pressure around us from the weight of the atmosphere above us at sea level.
So, if using an absolute pressure gauge, and I was standing at sea level, the gauge would read 14.696 PSIA. We usually round this to 15.
If I was using a gauge pressure gauge, at sea level,the pressure would read 0.
Mechanical gauges are the most common tool to sense this pressure. See the refrigerant gauge set below
Absolute pressure (PSIA) assumes we are in a complete vacuum. Many things in this business use absolute because it the basis of all pressure.
Gauge pressure (PSIG) starts at the pressure around us from the weight of the atmosphere above us at sea level.
So, if using an absolute pressure gauge, and I was standing at sea level, the gauge would read 14.696 PSIA. We usually round this to 15.
If I was using a gauge pressure gauge, at sea level,the pressure would read 0.
Mechanical gauges are the most common tool to sense this pressure. See the refrigerant gauge set below
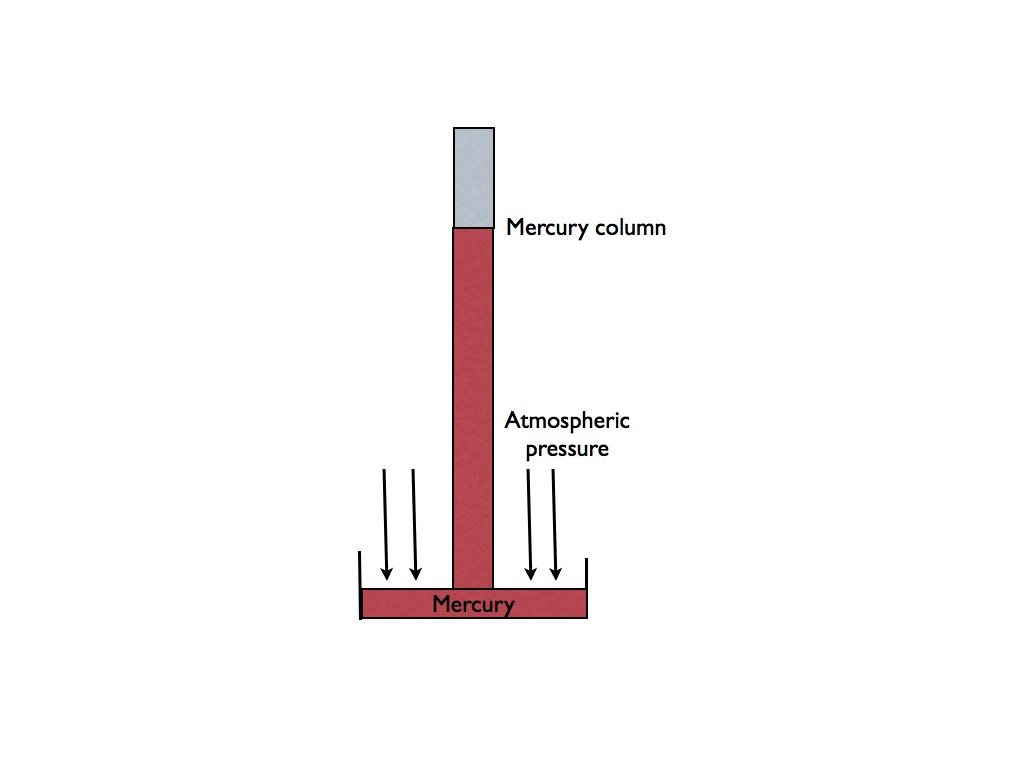
The mercury tube manometer
Pressures below atmospheric are measured using this device.
Here is the way it works. A glass tube with one end sealed off is filled with mercury. The open end is then placed in a pan filled with mercury. With the closed end up, the mercury finds its own height. Above the top of the mercury in the tube is a perfect vacuum. Therefore there is no pressure in the top of the tube that could push the mercury down.
There is however, atmospheric pressure outside the tube. This pressure pushes the mercury column down and up the tube. The mercury is quite heavy and will pull the column down.
Where the mercury weight and the atmospheric pressure balance, is the height of mercury in inches.
These devices are commonly used to measure barometric pressure for weather uses.
In our industry, atmospheric pressure is not involved inside the refrigeration system, so all pressure is
absolute.
Here is the way it works. A glass tube with one end sealed off is filled with mercury. The open end is then placed in a pan filled with mercury. With the closed end up, the mercury finds its own height. Above the top of the mercury in the tube is a perfect vacuum. Therefore there is no pressure in the top of the tube that could push the mercury down.
There is however, atmospheric pressure outside the tube. This pressure pushes the mercury column down and up the tube. The mercury is quite heavy and will pull the column down.
Where the mercury weight and the atmospheric pressure balance, is the height of mercury in inches.
These devices are commonly used to measure barometric pressure for weather uses.
In our industry, atmospheric pressure is not involved inside the refrigeration system, so all pressure is
absolute.
The manifold gauge set
The left gauge is a compound gauge. The zero point is actually 14.696 PSIA. The measurement below 0 is in a vacuum. Note the vacuum reaches 30 in Hg (mercury). That would be the mercury in the vertical tube would be at the same level as the mercury in the bowl. At standard atmospheric pressure at sea level, the mercury would be at the top of the tube or 0 in Hg.
So why do we in the industry care? To clear our systems of contaminants, we need to evacuate all gasses from our systems. This gauge tells us we are in a vacuum. Its accuracy in vacuum poor. There are other more accurate gauges for this work. However it tells us the system is in a vacuum.
The right gauge reads PSIG. Its zero point is 14.696 PSIA. For our purposes, we call this 0 # PSIG.
This gauge reads a higher pressure (500 #) opposed to 120 #) on the left gauge.
We will discuss these gauges further as we learn more.
So why do we in the industry care? To clear our systems of contaminants, we need to evacuate all gasses from our systems. This gauge tells us we are in a vacuum. Its accuracy in vacuum poor. There are other more accurate gauges for this work. However it tells us the system is in a vacuum.
The right gauge reads PSIG. Its zero point is 14.696 PSIA. For our purposes, we call this 0 # PSIG.
This gauge reads a higher pressure (500 #) opposed to 120 #) on the left gauge.
We will discuss these gauges further as we learn more.
The pressure-temperature relationship
When a substance changes from a liquid to a gas, the gas takes up more space than the liquid. An example is water. When water changes to a gas (steam) it takes up 1200 times the space that liquid water does. If the water is heated in an closed container it cannot expand the 1200 times. The pressure must then increase. If more heat is added, more water boils. The pressure must then increase more. At this point there is both water and steam in the container. Substances that exist in a container as both liquid and gas are called saturated. If heat is added the pressure increases. If heat is taken away, the pressure decreases. An example would be a steam boiler that had been heated to the point that the water boils. As the water boils, steam is formed and pressure increases. As more heat is added, more water boils and the pressure increases.
We can plot the various temperatures and the related pressures. There are charts that will tell you the temperature if you know the pressure and vice versa.
We can plot the various temperatures and the related pressures. There are charts that will tell you the temperature if you know the pressure and vice versa.
The can crusher
The above video illustrates the pressure-temperature relationship. When the water is boiling it fills the container and displaces the air. So at this point, the can contains only liquid water and steam. It is a saturated mix. When I remove the heat and place the cap on the can, I am isolating the mixture. There is nothing in the can but water and steam.
The heat source has ben removed so the temperature starts reducing to ambient temperature.
The heat source has ben removed so the temperature starts reducing to ambient temperature.
|
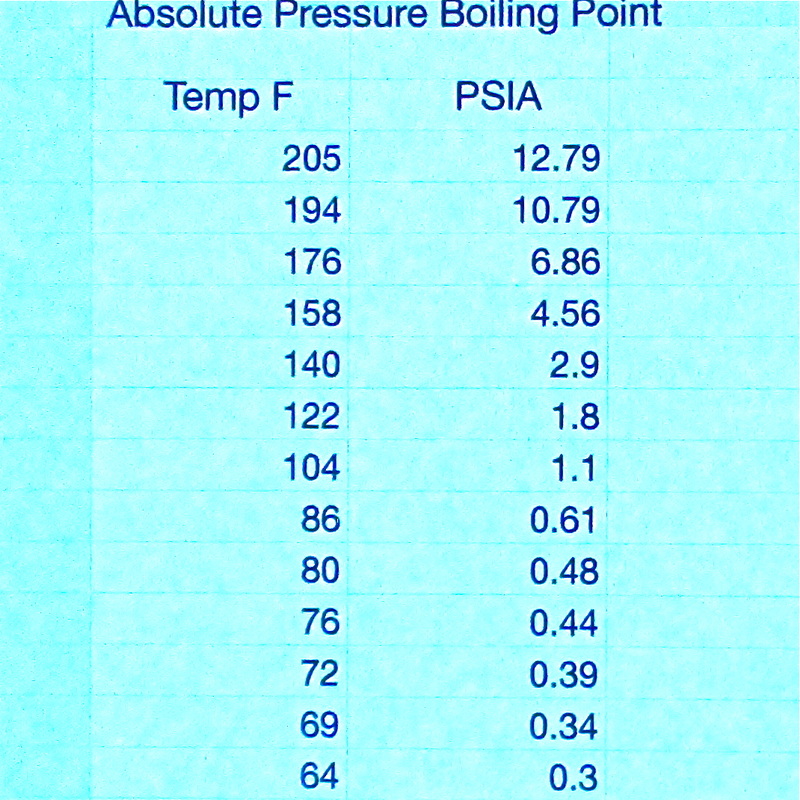
The chart on the left shows various boiling temperatures for water at various absolute pressures.
So when my can cools with the cap on, the pressure reduces to its saturated pressure. Note on the chart to the left, the temperature 72 degrees F. The pressure is .39 PSIA. That means that the pressure on the outside of the can is 14.696-.39= 14.3 PSI. If you multiply the number of square inches of the can surface area, 288*14.303=4119 psi, no wonder the can crushed! |
You know water will boil at 212 degrees F. What you may not know is that water boils at 212 degrees at atmospheric pressure. If I change the pressure above the water, I change the boiling point.
Below is a steam chart
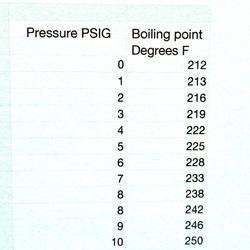
If the pressure on the water is 0 PSIG then it boils at 212 degreesF. However, if the pressure is increased to 10 PSIG then the boiling point is 250 degreesF.
As the pressure increases on any substance the boiling point will increase.
In a like manner, the boiling point will decrease if the pressure is decreased.
The chart on the left is only accurate if the container has only water and steam in it. If any other substance is inside with the water the chart will change.
Each chemical has different boiling points at different pressures.
So, how does this affect refrigeration?
We choose refrigerants on the basis of its boiling points at the different pressures. (There are many other factors involved but for our purposes, we will only use the pressures).
A good refrigerant would boil at a reasonable pressure for the temperatures we are using. An example would be R-22. This refrigerant has been used for a number of decades in air conditioners. Its boiling point is 40 degreesF at a pressure of 68.6#. Forty degrees is a good temperature for air conditioners because it is above freezing and low enough to provide the temperature difference to cool air at 75 degreesF. So now I have a pressure that works for cooling the inside of the structure.
Remember the latent heat? When the refrigerant boiled it absorbed the latent heat of vaporization of R-22. Now the heat is contained in the gas.
If I then move outside the temperature is, lets say, 90 degreesF. In order to have enough temperature difference between the outside air and the refrigerant for it to transfer heat we would need about 30 degrees difference. So that means the refrigerant must be 120 degreesF.
Take a look at this temp-press chart.
Find120 degreesF on the left. Move horizontally right to the R-22 column. Note the pressure at 260#.
In order to make this system work, the pressure on the refrigerant in the evaporator must be 68.6#. Also, the pressure in must be 260#. We need something that will change the the pressure.
As the pressure increases on any substance the boiling point will increase.
In a like manner, the boiling point will decrease if the pressure is decreased.
The chart on the left is only accurate if the container has only water and steam in it. If any other substance is inside with the water the chart will change.
Each chemical has different boiling points at different pressures.
So, how does this affect refrigeration?
We choose refrigerants on the basis of its boiling points at the different pressures. (There are many other factors involved but for our purposes, we will only use the pressures).
A good refrigerant would boil at a reasonable pressure for the temperatures we are using. An example would be R-22. This refrigerant has been used for a number of decades in air conditioners. Its boiling point is 40 degreesF at a pressure of 68.6#. Forty degrees is a good temperature for air conditioners because it is above freezing and low enough to provide the temperature difference to cool air at 75 degreesF. So now I have a pressure that works for cooling the inside of the structure.
Remember the latent heat? When the refrigerant boiled it absorbed the latent heat of vaporization of R-22. Now the heat is contained in the gas.
If I then move outside the temperature is, lets say, 90 degreesF. In order to have enough temperature difference between the outside air and the refrigerant for it to transfer heat we would need about 30 degrees difference. So that means the refrigerant must be 120 degreesF.
Take a look at this temp-press chart.
Find120 degreesF on the left. Move horizontally right to the R-22 column. Note the pressure at 260#.
In order to make this system work, the pressure on the refrigerant in the evaporator must be 68.6#. Also, the pressure in must be 260#. We need something that will change the the pressure.
The compressor

How do we change the pressure? Well we just use a compressor.
The compressor is considered the prime mover of the mechanical refrigeration system.
We will discuss compressors and how they work later on.
For now we will look at the compressor as an air pump that can change gas pressure from low to high.
The compressor is considered the prime mover of the mechanical refrigeration system.
We will discuss compressors and how they work later on.
For now we will look at the compressor as an air pump that can change gas pressure from low to high.
For further explanation of compressors, click here
The evaporator
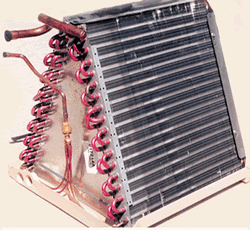
The evaporator is a set of small tubes with fins attached. The fins extend the surface area of the tubes to allow a better heat exchange. A fan blows across the assembly to cool the air. On an air conditioner, the temperature of the air is dropped about 20 degreesF.
The evaporator is placed on the suck side of the compressor. The pressure drops to the 68.6# and the refrigerant boils and absorbs latent heat into the refrigerant. The vapor then moves into the compressor.
The evaporator is placed on the suck side of the compressor. The pressure drops to the 68.6# and the refrigerant boils and absorbs latent heat into the refrigerant. The vapor then moves into the compressor.
For a further explanation of evaporators click here
The condenser

The high pressure gas comes out of the compressor and enters the condenser. The condenser is very similar to the evaporator. The hot gas from the compressor is condensed at high pressure. In our example, the pressure is raised to 260# by the compressor and the latent heat that was absorbed by the evaporator is released at 120 degreesF.
For more about condensers, click here
The expansion device

The last necessary part is the expansion device.
If the compressor is the prime mover, the expansion device is the prime controller. It is located between the outlet of the condenser and the inlet of the evaporator. Where the compressor increases the pressure, the expansion device lowers the pressure. Expansion devices vary from a simple small diameter capillary tube to electronically controlled valves.
They all perform a special function. They limit the amount of refrigerant that can pass through.
The expansion device's job may be the most critical of all the parts because they control flow.
If the compressor is the prime mover, the expansion device is the prime controller. It is located between the outlet of the condenser and the inlet of the evaporator. Where the compressor increases the pressure, the expansion device lowers the pressure. Expansion devices vary from a simple small diameter capillary tube to electronically controlled valves.
They all perform a special function. They limit the amount of refrigerant that can pass through.
The expansion device's job may be the most critical of all the parts because they control flow.